태양에너지만으로 고효율 암모니아 생산
기사입력 2024.04.16 16:03
.jpg)
▲이번 연구를 주도한 (윗줄 왼쪽부터)제 1저자 아마드 타예비 연구원, 장성연, 장지욱 교수, (아랫줄 왼쪽부터)제 1저자 김지은, 제 1저자 라시미, 제 1저자 무히불라 알 무바록 연구원, 오동락 연구원
외부전압 없이 생산, 다양한 친환경 연료 생산 활용
태양에너지만으로 고효율의 암모니아를 생산할 수 있는 기술이 개발됐다.
UNIST(총장 이용훈) 에너지화학공학과 장지욱, 장성연 교수팀은 스탠퍼드 대학교 Thomas F. Jaramillo 교수와 공동으로 페로브스카이트 기반 친환경 암모니아 생산 광전극 시스템을 개발했다고 16일 밝혔다.
미국 에너지부가 정한 친환경 암모니아 생산 속도의 상용화 기준치를 약 1.7배 웃돌았다. 세계 최고 기록이다.
태양에너지로 폐수의 주요 성분인 질산염(NO3-)을 물속에서 환원해 암모니아(NH3)를 생산하는 원리다. 개발한 시스템은 세계 최고의 태양광-암모니아 전환 속도를 기록했으며 물에 약한 페로브스카이트의 단점을 극복했다.
공업 원료인 암모니아는 비료, 음식, 제약 등 고부가 가치 화합물을 합성하는 데 쓰인다. 현재 암모니아의 대부분은 ‘하버-보쉬 공정’으로 생산된다. 반면에 친환경적이지 않고 막대한 양의 화석연료를 소모해야 된다는 문제가 있다.
연구팀은 페로브스카이트 태양전지를 보호해 높은 성능과 내구성을 갖춘 광전극 시스템과 루세늄을 티타늄 나노시트에 올린 고성능 암모니아 생산 촉매를 개발했다.
페로브스카이트는 빛을 잘 흡수해 전하를 많이 만들 수 있지만 물에 쉽게 분해된다. 연구팀은 페로브스카이트를 쉽게 액체가 되는 필즈금속으로 보호하는 동시에 고성능 암모니아 생산 촉매와 강하게 결합시켰다. 필즈금속은 녹는 온도가 63도로 낮아 쉽게 녹고 상온에서는 고체가 돼 이러한 설계가 가능하다.
제작된 광전극은 페로브스카이트가 물과 직접 접촉하는 것을 막는다. 페로브스카이트와 암모니아 생산 촉매를 전기적으로 연결하고 고정한다. 물속에서 빛을 받은 광전극은 전하를 생산한다. 전하는 전극 표면에 노출된 암모니아 생산 촉매에 효율적으로 전달돼 안정적으로 높은 효율의 암모니아가 만들어진다.
연구팀은 암모니아를 외부 전압없이 생산하기 위해 물보다 낮은 전압에 반응하는 글리세롤을 이용했다. 먼저, 백금 촉매를 티타늄 나노시트에 올려 글리세롤의 산화 반응속도를 높였다.
광전극에서 생산되는 전압과 글리세롤의 산화반응을 통해 암모니아 전환에 필요한 전압의 최소량을 맞췄다. 즉, 글리세롤이 첨가된 물에 광전극을 담그고 빛을 쬐면 자발적으로 암모니아를 생산할 수 있는 것이다. 암모니아 생산과 동시에, 부산물로 글리세롤보다 9배 이상 가치 높은 글리세릭 엑시드도 만들었다.
개발된 광전극으로 암모니아를 생산하는 속도는 최대 1,745μgNH3 cm⁻²h⁻¹를 기록했다. 이는 미국 에너지부가 정한 친환경 암모니아 생산 속도 상용화 기준인 1,000μgNH3 cm⁻²h⁻¹를 훨씬 넘어섰다.
개발된 시스템에 사용된 질산염 환원 촉매를 다른 종류로 바꾸면 다양한 고부가가치 물질의 생산에도 활용할 수 있다.
장지욱 교수는 “이번 연구를 통해 폐수의 주성분인 질산염과 바이오디젤의 부산물인 글리세롤을, 암모니아와 고부가 가치의 글리세릭 엑시드를 생산했다”며 “개발된 기술은 외부전압 없이도 고효율 암모니아를 생산할 수 있으며, 다양한 친환경 연료를 생산하는데도 활용할 수 있을 것”이라고 설명했다.
장성연 교수는 “본 연구는 고효율 태양광 연료 생산에 응용한 매우 중요한 연구다”며 “태양광 연료의 상용화가 되는 태양광 연료 생산 속도 기준을 초과 달성했다는 점에서 큰 의의가 있다”고 설명했다.
이번 연구 성과는 촉매 연구에서 가장 탑 저널인 ‘네이처 캐탈리시스(Nature Catalysis)’에 4월 1일자로 공개됐다. 아마드 타예비(Ahmad Tayyebi) 박사후 연구원, 라쉬미 메흐로트라(Rashmi Mehrotra), 무히불라 알 무바록(Muhibullah Al Mubarok), 김지은 UNIST 석박통합과정 대학원생이 공동 1저자로 참여했다.
연구 수행은 과학기술정보통신부 한국연구재단의 우수연구자교류지원사업(Brainlink)과, 글로벌 기초연구실 지원사업 (BRL) 등을 받아 이뤄졌다.
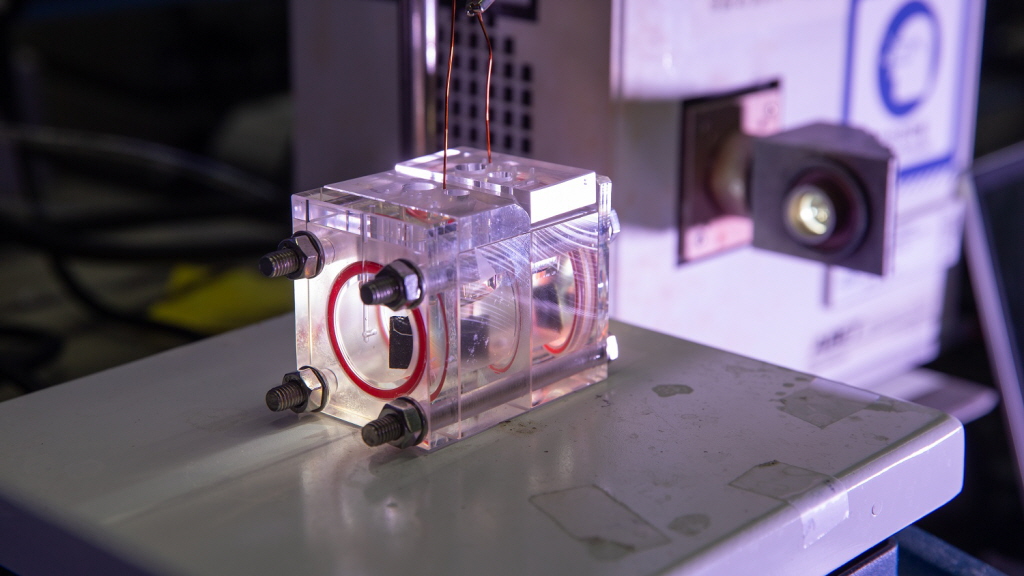
▲제작된 페로브스카이트 광전극 시스템의 실제 모습
많이 본 뉴스
[열린보도원칙] 당 매체는 독자와 취재원 등 뉴스이용자의 권리 보장을 위해 반론이나 정정보도, 추후보도를 요청할 수 있는 창구를 열어두고 있음을 알려드립니다.
고충처리인 장은성 070-4699-5321 , news@e4ds.com