한국전자기술연구원(KETI, 원장 신희동)이 원자 단위 수전해 촉매 합성에 성공해 희귀 금속 사용량을 획기적으로 줄이고 성능을 높여 향후 그린 수소 생산을 위한 기폭제가 될 것으로 기대된다.
.jpg)
▲이번 연구(논문)의 제1저자인 박진우 연구원(좌)과 교신저자인 임현수 책임연구원(우)
희귀 금속 사용량 획기적 감소, 성능↑·가격 ↓
한국전자기술연구원(KETI, 원장 신희동)이 원자 단위 수전해 촉매 합성에 성공해 희귀 금속 사용량을 획기적으로 줄이고 성능을 높여 향후 그린 수소 생산을 위한 기폭제가 될 것으로 기대된다.
KETI는 수전해 기술에 필수적인 고가의 금속 촉매 사용을 대폭 절감할 수 있는 단원자 촉매 기술을 개발했으며, 관련 내용이 화학공학 분야 국제 저명 학술지에 게재됐다고 4일 밝혔다.
수전해 기술은 전기에너지를 통해 물을 수소와 산소로 분리하는 과정으로, 탄소 배출이 전혀 발생하지 않는 그린수소를 생산하는 청정에너지 기술로 알려져 있다.
현재 물에 전기 반응을 일으키는 촉매 소재의 경우 백금(Pt)·이리듐(Ir) 등 희귀 금속과, 니켈(Ni)·코발트(Co) 등의 핵심 광물이 주로 사용됐으나 높은 가격과 수급 부족 등의 문제가 꾸준하게 제기됐다.
KETI 신재생에너지연구센터 연구진이 개발에 성공한 단원자 촉매는 금속 함유량이 40∼70%에 달하는 기존 촉매에 비해 약 1%만의 금속만으로도 동일한 성능과 내구성을 갖췄다는 것이 가장 큰 특징이다.
KETI 연구진은 극소량(촉매 성분의 1%)의 니켈에 특정 유기물(도파민)을 중합하여 니켈이 원자 단위로 존재하는 환경을 조성했으며, 열분해 등 일련의 화학적 공정을 진행하여 고효율의 단원자 촉매를 합성했다.
기술 개발을 주도한 임현수 책임연구원(박사)과 박진우 연구원(박사과정)에 따르면 단원자 촉매는 원자 덩어리로 구성된 벌크(Bulk) 촉매에 비해 입자 하나의 촉매 활용도가 이론상 100%에 가깝기 때문에 소량의 금속만으로도 상용 촉매만큼의 성능을 보여줬다.
또한 단원자 촉매로 인해 고가의 금속 사용량을 대폭 절감한 만큼 수전해 촉매 제작을 위한 가격 경쟁력이 향상될 것으로 기대된다.
KETI 미래전략기술 분야 기본연구사업 그리고 한국연구재단 국가핵심소재연구단(나노 및 소개기술개발사업)을 통해 추진된 이번 연구 결과는 화학공학 분야의 세계적인 학술지 ‘Chemical Engineering Journal(IF=16.744)’ 최신호(468호)에 ‘수소 발생 반응을 위한 다공성의 니켈 단원자 촉매 개발’의 제목으로 게재되는 성과를 거뒀다.
KETI 신재생에너지연구센터 박노창 센터장은 “단원자 촉매는 금속 사용량을 획기적으로 절감할 수 있다는 점에서 진정한 의미의 그린수소 실현에 기여할 수 있다”며 “센터는 향후 단원자 촉매 기술의 상용화를 적극 추진하여 친환경 에너지 확산을 위한 미래 기반을 마련하겠다”고 밝혔다.
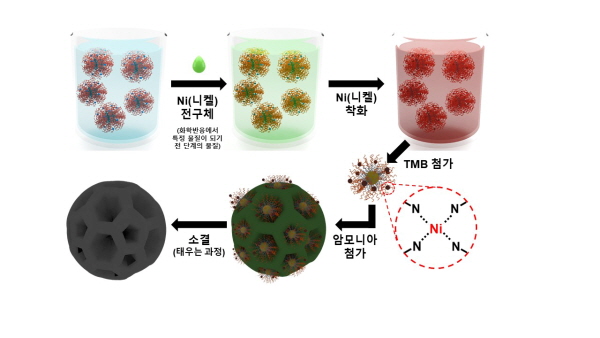
▲1%의 니켈(Ni)과 기타 화합물이 포함된 특수 용액에 TMB(1,3,5-트리메틸벤젠) 성분을 추가하여 니켈이 원자 단위로 존재하는 환경을 조성한 뒤, 열분해 등의 화학 공정을 통해 니켈-단원자 촉매를 합성했다.
.jpg)
