UNIST(총장 이용훈) 에너지화학공학과 이현욱, 정성균 교수팀과 KAIST 서동화 교수팀은 상온에서도 구동하는 전고체 나트륨 이차전지를 개발하며, 값싼 친환경 소재인 프러시안계 물질로 고체 전해질의 비싼 가격과 환경문제 등을 동시에 해결해 전고체 전지 상용화를 크게 앞당겼다.
UNIST 이현욱 교수팀, 프러시안계 물질이용 고체 전해질 개발
값싼 친환경 소재인 프러시안계 물질로 고체 전해질을 개발했다. 고체 전해질의 비싼 가격과 환경 문제 등을 동시에 해결해 전고체 이차전지 상용화를 크게 앞당길 것으로 기대된다.
UNIST(총장 이용훈) 에너지화학공학과 이현욱, 정성균 교수팀과 KAIST 서동화 교수팀은 상온에서도 구동하는 전고체 나트륨 이차전지를 개발했다고 26일 밝혔다.
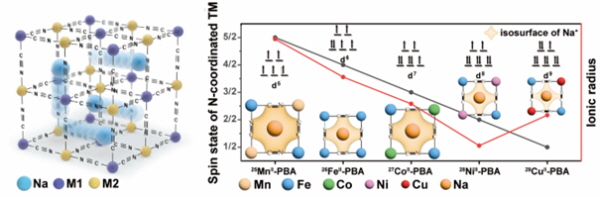
▲프러시안 블루계 물질의 구조 모식도와 전이 금속 크기에 따른 이온 채널 크기 모식도
친환경 물질인 프러시안계 물질(Prussian Blue analogues, PBAs)을 고체 전해질로 사용했다. 프러시안계 물질은 18세기부터 사용된 청색 염료 물질 중 하나로 청바지의 염료로 사용된다.
친환경 물질인 PBAs는 나트륨 이차전지의 핵심소재인 양극활물질(양극재)로 흔히 쓰인다. 이온이 이동할 수 있는 넓은 이온 전도 채널을 가지며 쉽게 합성 가능하다. 구조적으로 안정하고 값 또한 싸다. 이런 장점과 더불어 전이 금속에 따라 그 특성까지 달라져 많은 주목을 받고 있다.
연구팀은 PBAs의 고유 특성이 이온 전도도를 높일 수 있다고 판단했다. 전이 금속의 종류를 변경하면서 이온 전도의 변화 추이를 관찰했다. 이를 통해 전이 금속의 크기에 따라 이온 채널의 크기가 달라지는 것을 확인했다. 큰 이온 채널을 가진 물질은 높은 이온 전도성을 보이는 것이다.
각 양극, 음극활물질과의 계면 안정성 분석을 통해 적절한 물질 군을 선별했다. 전이금속 이온의 종류에 따라 이온전도도와 계면 안정성에 차이를 보였다. 연구팀은 결과를 바탕으로 망간계 프러시안 블루 계열 물질을 통해 전고체 나트륨 이차전지를 개발했다. 전지는 상온에서도 0.1mS/Cm 급의 나트륨 이온전도도를 보이며 고체 전해질로서의 가능성을 입증했다. 대기 안정성이 우수하고, 상온 제작공정이 가능해 기존의 황화물전해질, 산화물전해질의 단점을 모두 극복한 새로운 고체 전해질을 개발한 것이다.
제 1저자 김태원 에너지화학공학과 연구원은 “본 연구를 통해 친환경 물질인 프러시안 블루계 물질을 고체 전해질로 적용했다”며 “앞으로 고체 전해질의 새로운 시각을 제시한 연구다”고 설명했다.
제 1저자 안상혁 연구원은 “본 연구를 통해 기존의 고체 전해질의 비싼 가격과 환경 문제에 대한 고질적 문제를 해결할 수 있었다”며 “앞으로 전고체 전지 상용화를 앞당길 수 있는 연구다”고 설명했다.
또한 에너지화학공학과 이현욱 교수는 “기존 황화물, 산화물, 할라이드계 고체전해질에 제한된 연구분야가 새로운 소재 발견으로 가능성이 확대되기 바란다”며 “이번 연구는 이런 부분을 지적하면서 동시에 새로운 해결방안을 성공적으로 제시할 수 있는 연구였다”고 설명했다.
이번 연구는 UNIST 미래 선도형 특성화 사업, 과학기술정보통신부·한국연구재단 중견연계 신진후속 사업의 지원으로 수행됐다. 연구 결과는 에너지·재료 분야 국제학술지 앙게반떼 케미(Angewandte Chemie international edition)에 8월28일 자로 온라인 게재됐다.

▲(왼쪽부터)이현욱 교수, 제 1저자 안상혁 연구원, 제 1저자 김태원 연구원, 제 1저자 송유엽 연구원

