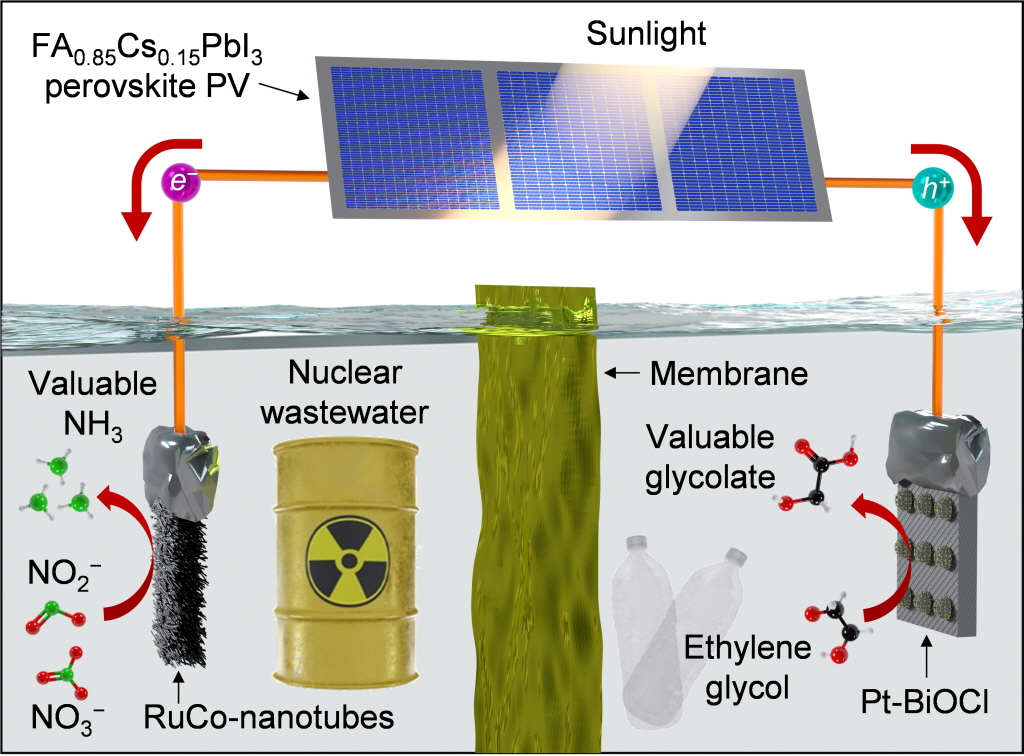
UNIST 신소재공학과 조승호·송명훈 교수 연구팀은 태양광 전기를 활용하여 이산화탄소 배출 없이 암모니아를 합성하고, 동시에 폐플라스틱에서 고부가가치 물질인 글리콜산을 생산하는 새로운 광전기화학 시스템을 개발했다.
UNIST, 아질산염 태양광 에너지 환원 암모니아 변환
폐수와 폐플라스틱에서 암모니아와 화장품 원료로 쓰이는 글리콜산을 생산할 수 있는 혁신적인 기술이 개발됐다.
UNIST 신소재공학과 조승호·송명훈 교수 연구팀은 태양광 전기를 활용하여 이산화탄소 배출 없이 암모니아를 합성하고, 동시에 폐플라스틱에서 고부가가치 물질인 글리콜산을 생산하는 새로운 광전기화학 시스템을 개발했다고 밝혔다.
암모니아는 전 세계적으로 황산 다음으로 많이 생산되는 필수 화합물이지만, 기존 생산 공정인 하버·보슈법은 이산화탄소 배출이 많아 지속가능한 대안 기술 개발이 요구되어 왔다.
이번 연구팀이 개발한 시스템은 폐수 속 아질산염(NO₂⁻)을 태양광 에너지로 환원해 암모니아로 변환하며, 동시에 음극에서는 폐플라스틱에서 얻은 에틸렌글리콜을 글리콜산으로 산화시키는 짝 반응을 수행한다.
연구팀은 에너지 효율과 생산 속도 면에서 세계 최고 수준을 기록했다. 양극 기준 에너지 효율은 52.3%로 보고된 최고치를 경신했으며, 암모니아 생산 속도는 146μ㏖/㎠h로 미국 에너지부의 상용화 기준을 훨씬 상회했다. 이는 기존 기록보다 46% 향상된 수치다.
또한, 연구팀은 폐수 속 질산염과 아질산염 중 에너지가 덜 소모되는 아질산염을 선택적으로 환원하도록 설계된 촉매(RuCo-NT/CF)를 개발했다.
기존 에너지 소모가 많은 산소 발생 반응 대신 글리콜산 발생 반응을 활용하여 필요한 전기에너지를 더욱 절감하는 데 성공했다.
해당 시스템에는 기존 실리콘 태양전지보다 높은 효율을 가진 페로브스카이트 태양전지가 사용되었으며, 이를 통해 광전류 밀도를 증가시켜 암모니아 생산 속도를 더욱 높일 수 있었다.
연구팀은 저준위 방사성 폐수와 페트병 추출물을 이용해 상용화 가능성을 검증했으며, 해당 조건에서도 높은 생산 효율을 확인했다.
송명훈 교수는 “이번 연구는 이산화탄소를 배출하지 않으면서 암모니아와 글리콜산을 생산할 수 있는 지속 가능한 기술의 가능성을 보여준 중요한 성과”라고 강조했다.
조승호 교수는 “태양광과 폐기물을 활용해 탄소중립형 에너지 솔루션을 제시했다는 점에서 의의가 크다”고 밝혔다.
이번 연구는 과학기술정보통신부의 기초연구실 지원 사업을 통해 수행됐으며, 연구 결과는 2월19일 국제 학술지 나노 레터스(Nano Letters)*에 게재됐다.