기초과학연구원(IBS, 원장 노도영) 다차원 탄소재료 연구단 로드니 루오프 연구단장 연구팀은 갈륨, 철, 니켈, 실리콘으로 구성된 액체 금속 합금을 이용하여 1기압에서 다이아몬드를 합성하는 데 세계 최초로 성공했다.
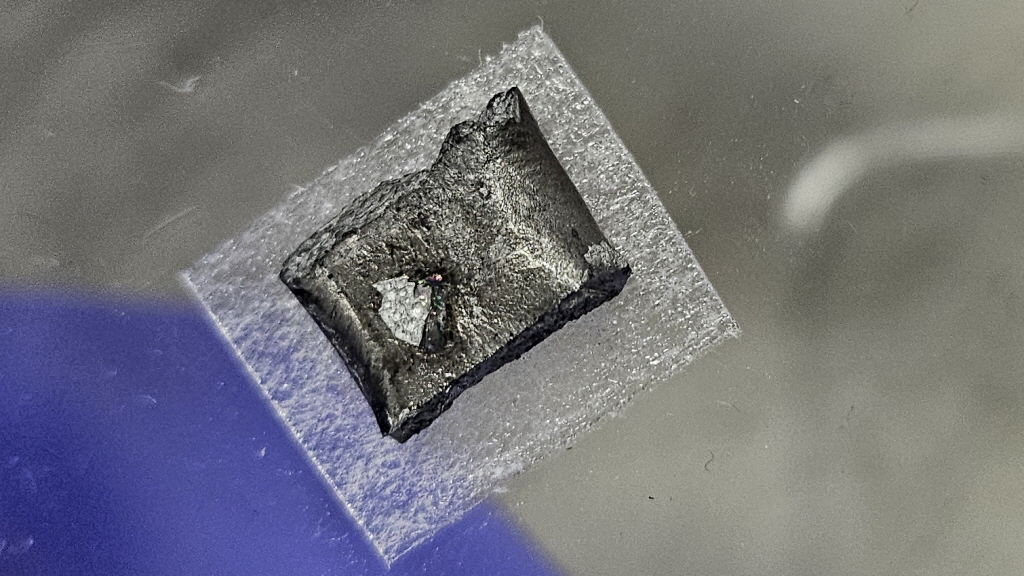
▲RSR-S 장치를 통해 만들어진 다이아몬드 결정
‘RSR-S’ 이용 1,025℃·1기압서 15분만에 합성
나노 크기 자기센서·양자 컴퓨터 등 응용 기대
대부분의 다이아몬드는 고온고압 조건에서 생산된다는 패러다임이 완전히 깨졌다. 바로, 우리 주변 기압인 대기압(1기압)에서 다이아몬드를 합성시키는 방법이 최초 개발된 것이다.
기초과학연구원(IBS, 원장 노도영) 다차원 탄소재료 연구단 로드니 루오프 연구단장 연구팀은 갈륨, 철, 니켈, 실리콘으로 구성된 액체 금속 합금을 이용하여 1기압에서 다이아몬드를 합성하는 데 세계 최초로 성공했다고 25일 밝혔다.
다이아몬드는 우수한 열 전도성과 단단함 및 내화학성을 갖는 탄소 물질로 전자기기의 열 전도체, 반도체의 온도 상승을 방지하는 방열 장치 등 활용도가 매우 높다.
반면에 이런 다이아몬드를 합성하기란 상당히 까다롭다.
대부분의 다이아몬드는 섭씨 1,300도∼1,600도에 육박하는 고온과 표준 대기압(1기압)의 5만 배∼6만 배에 달하는 고압 조건에서만 합성되기 때문이다.
또한 고온고압 조건을 유지하기 위한 압력 셀의 크기 제한 때문에 합성 가능한 다이아몬드의 크기는 약 1세제곱센티미터로 제한된다.
IBS 연구팀은 이러한 기존 다이아몬드 합성 패러다임을 완전히 깨는, 1,025도 온도 및 1기압 압력 조건에서 다이아몬드를 최초 합성했다.
우선 연구팀은 빠르게 가열 및 냉각 가능한 ‘RSR-S’이라는 장치를 자체 제작해, 3시간이 걸리는 기존 장치들과 달리 총 15분이면 모든 실험 준비 과정이 완료될 수 있게 했다.
RSR-S 장치는 온도와 압력을 빠르게 조절해 액체 금속 합금을 만드는 장치로, 다이아몬드를 성장시킬 수 있는 최적의 온도, 압력, 액체 금속 합금 비율 조건을 찾기 위해 수백 개의 매개변수 조정에 사용됐다.
연구팀은 메탄과 수소에서 갈륨 77.75%, 니켈 11.00%, 철 11.00%, 실리콘 0.25%로 구성된 액체 금속 합금을 만들었다.
그리고 하부 표면에서, 다이아몬드 구성 물질인 탄소가 확산되는 것을 확인했다.
액체 금속 합금 하부에서 탄소 확산이 1,025도의 온도와 1기압 압력에서 이루어짐으로써 다이아몬드가 성장한다는 사실을 밝힌 것이다.
또한 ‘광 발광 분광법’이라는 실험을 통해 물질에 빛을 쏘아 방출되는 파장 빛을 분석해봄으로써 다이아몬드 내 ‘실리콘 공극 컬러 센터’ 구조를 발견했다.
이 구조는 액체 금속 합금의 구성요소 중 하나인 실리콘이, 탄소로만 이루어진 다이아몬드 결정 사이에 끼어들어 있는 구조다.
이때 실리콘 공극 컬러 센터 구조는 양자 크기의 자성을 가져 자기 민감도가 높고, 양자 현상(양자적인 특성)을 띈다. 그래서 향후, 나노 크기의 자기 센서 개발과 양자 컴퓨터 분야로 응용이 기대된다.
공동 교신저자 성원경 연구위원은 “이번 연구 결과를 바탕으로, 보다 쉽고 크게 다이아몬드를 만들 수 있게 됐다. 액체 금속 합금의 구성을 다른 금속으로 대체하는 방법을 찾아 더욱 폭넓은 실험 조건에서 다이아몬드를 합성할 길을 열 것”이라며 후속 연구에 대한 기대를 밝혔다.
연구를 이끈 로드니 루오프 연구 단장은 “반도체, 기계 산업과 같은 주요 산업에 바로 접목할 수 있는 다이아몬드 합성 원천기술을 획득했다. 한국이 앞으로 빠르게 응용 분야를 확장해 관련 산업을 선도할 수 있을 것으로 기대된다”고 밝혔다.
연구 결과는 4월25일 0시(한국시간) 세계 최고 권위 국제학술지 ‘네이처(Nature, IF 64.8)’온라인판에 실렸다.
