한국전기연구원(KERI) 차세대전지연구센터의 하윤철 박사팀이 전고체전지용 황화물계 고체전해질을 더 빠르고 품질 좋게 만드는 ‘업그레이드형 공침법’을 개발했다.
▲업그레이드형 공침법을 개발한 KERI 하윤철 박사(왼쪽) 및 KAIST 변혜령 교수
전기연구원, 하윤철 박사팀, 업그레이드형 공침법 개발
전고체전지용 고체전해질을 더 빠르고 품질 좋게 만들 수 있는 방법이 개발돼 향후 전고체전지 상용화에 기여할 것으로 기대된다.
한국전기연구원(KERI) 차세대전지연구센터의 하윤철 박사팀이 전고체전지용 황화물계 고체전해질을 더 빠르고 품질 좋게 만드는 ‘업그레이드형 공침법’을 개발했다.
전고체전지는 전해질을 액체가 아닌 고체로 대체하여 화재나 폭발의 위험성을 크게 낮춘 차세대 배터리 기술이다.
하윤철 박사팀은 2021년에 고가의 황화리튬(Li2S) 없이도 원료를 한꺼번에 용기에 넣어 대량으로 제조할 수 있는 공침법을 개발해 주목받았으며, 이 기술은 ㈜대주전자재료에 이전됐다.
이후에도 KERI는 한국과학기술원(KAIST), ㈜대주전자재료와 협력하여 공침 현상의 메커니즘을 규명하고, 고체전해질 생산 시간 단축과 품질 향상을 위한 업그레이드형 공침법을 개발했다.
공침법은 원료를 용액에 녹여내고 침전시킨 후 필터로 걸러내는 과정이 핵심이다.
연구팀은 리튬과 황, 촉매를 적정 비율로 혼합하여 리튬의 순차적인 용해 과정과 중간 생성물을 분석하고, 이를 고체전해질 합성 공정에 적용하여 다양한 원료가 빠르고 균질하게 용해·공침되도록 최적화했다.
KAIST 변혜령 교수팀은 리튬의 용해 과정에서 발생하는 중간 생성물의 화학적 분석을 주도했으며, 같은 대학 백무현 교수팀과 POSTECH 서종철 교수팀의 도움을 받아 정확한 분자 구조를 규명했다.
이를 바탕으로 ㈜대주전자재료는 실제 고체전해질 양산 공정에 관련 기술을 접목했다. 그 결과, 고체전해질 생산 시간을 14시간에서 4시간으로 대폭 줄이는 업그레이드형 공침법이 탄생했다.
업그레이드형 공침법으로 합성된 고체전해질은 품질도 향상됐다. 양산화 과정에서 낮은 이온전도도를 보였던 기존 제조법과 달리, 이 공법을 적용한 고체전해질은 이온전도도가 5.7mS/cm로 액체전해질(약 4mS/cm)을 넘어섰다.
또한 700mAh 용량의 전고체전지 파우치셀에 적용하여 상용 리튬이온전지보다 높은 에너지 밀도(352Wh/kg)를 달성했으며, 1,000회 충·방전 후에도 80% 이상의 용량을 유지하여 안정적인 수명을 확인했다.
이번 연구 결과는 에너지 분야 국제 저명 학술지 ‘에너지 스토리지 머티리얼스(Energy Storage Materials)’에 게재됐으며, 연구진은 이 기술이 다양한 기능성 코팅막 제조에도 적용될 수 있음을 확인하고 특허 출원을 마쳤다.
하윤철 박사는 “이번 성과는 공침법의 원리를 상세히 분석해 최적화를 실현한 결과물”이라며 “전고체전지를 저렴한 비용으로 대량생산하는 시대를 열 것”이라고 밝혔다.
한편 KERI는 과학기술정보통신부 국가과학기술연구회 산하 정부출연연구기관이며, 이번 연구는 KERI 기본사업 및 산업부 소재부품기술개발사업으로 진행됐다.
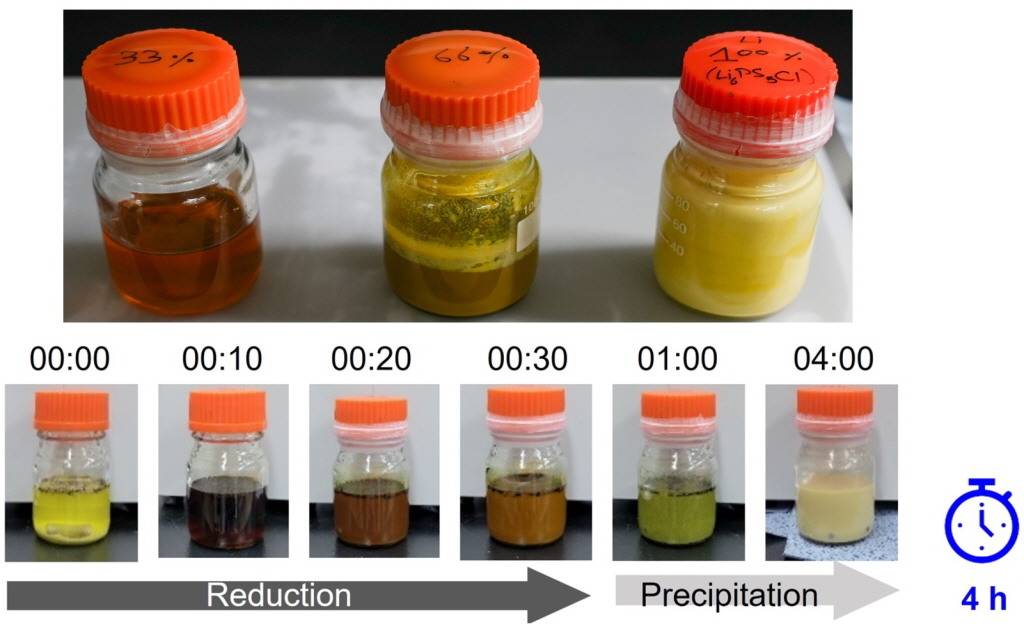
▲KERI 하윤철 박사팀이 리튬과 황, 촉매, 오황화인 및 염화리튬 원료를 적정 비율로 혼합하여 리튬의 순차적인 용해 정도에 따라 중간산물들이 연속적으로 형성되고 공침되는 과정을 분석했다.

