UNIST(총장 박종래) 신소재공학과 이승걸 교수팀이 저가의 비백금계 금속 촉매를 이용한 새로운 음이온 교환막 수전해 기술을 제시하며, 수소 생산 음이온 교환막 방식에서 발생하는 이오노머의 열화와 산화 현상을 예방할 수 있는 원리를 밝혔다.
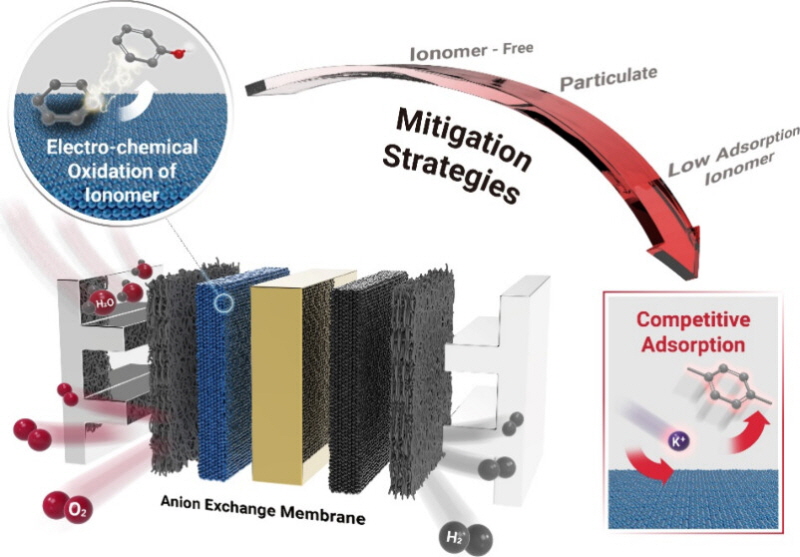
▲이오노머 전기화학적 산화 방지 전체 전략 모식도
UNIST, 이오노머 열화와 산화 예방해 수소 생산 효율 개선
백금이 필요없는 값싼 촉매를 이용해 수소 생산 장치의 성능과 내구성을 높일 수 있는 기술이 개발돼 향후 저비용 그린수소 생산에 도움을 줄 것으로 기대된다.
UNIST(총장 박종래) 신소재공학과 이승걸 교수팀은 저가의 비백금계 금속 촉매를 이용한 새로운 음이온 교환막 수전해 기술을 제시했다.
칼륨이 촉매 표면에 붙게 만들어 이오노머와 직접적으로 닿는 것을 줄이는 것이다. 이오노머가 산화되는 것을 막으면 수소 생산 비용을 절감할 수 있다.
일반 수소 생산 장치에서는 시간이 지나면서 이온 물질을 전달하는 이오노머의 성질이 변해 약해지기 쉽다. 이는 수소 생산 효율 저하와 장치의 수명 단축을 초래했다.
연구팀은 칼륨의 흡착 에너지가 유기 화합물보다 3배 이상 크다는 점을 활용했다. 수산화칼륨, 수산화나트륨 같은 물질이 음이온 교환막 수전해 시스템의 성능과 안정성을 높일 수 있음을 밝혀낸 것이다.
양이온 물질이 촉매 표면에 흡착해 이오노머와 촉매의 직접적인 접촉을 줄였다. 결국 이오노머의 산화를 막아 수소 생산 성능을 유지할 수 있음을 물질의 전자 구조를 계산하는 밀도범함수이론(DFT)을 통해 입증했다.
기존에도 염기성이 강한 수산화칼륨과 수산화나트륨 수용액을 이용해 성능을 개선하려는 시도는 있었으나, 그 구체적인 원리가 밝혀지지 않았다. 그러나 이번 연구에서 규명된 경쟁적 흡착 전략은 저가 촉매의 상용화 가능성을 한층 높일 것으로 전망된다.
제1저자 임지훈 연구원은 “경쟁적 흡착 전략이 촉매와의 접촉면에서 발생하는 이오노머 소재의 전기화학적 산화를 줄이는 데 효과적”이라고 강조했다.
이승걸 교수는 “이번 연구가 고성능 알칼리 음이온 교환막 수전해 시스템을 비롯한 다양한 에너지 장치의 성능과 안정성을 개선하는 방향성을 제시할 것”이라고 말했다.
연구 결과는 세계적인 에너지 분야 학술지인 ACS Energy Letters 지에 6월2일 온라인으로 실렸다. 연구는 미국 로스 알라모스 국립 연구소의 Yu Seung Kim 박사 연구팀, 미국 버클리 대학과 버클리 랩의 Shannon Boettcher 교수와 공동으로 수행됐으며, 미국 에너지부와 한국연구재단의 지원을 받았다.
