
▲UNIST 조재흥 교수팀
조재흥 교수팀, 촉매 합성·유기기질 산화 반응 원리 규명
화합물에서 전자를 뺏어오는 산화력이 우수한 촉매가 개발됐다. 합성 화학뿐 아니라 금속 촉매 개발 등 다양한 분야의 초석이 될 것으로 기대된다.
UNIST(총장 이용훈) 화학과 조재흥 교수팀은 새로운 망간-플루오린 촉매 합성에 성공했다고 20일 밝혔다.
이온이나 분자가 원자 주위를 둥그렇게 둘러싸고 결합하는 거대고리 피리디노판 배위 시스템을 활용했다.
개발된 촉매를 활용하면 유독성 물질인 ‘톨루엔 유도체’가 전자를 쉽게 잃도록 만드는 ‘산화’ 반응을 유도할 수 있다는 것 또한 확인했다.
조재흥 교수는 “강한 탄소-수소 결합을 가진 유기물이 활성화될 수 있었던 것은 높은 환원 전위를 지닌 망간-플루오린 종의 특징 때문이다”고 설명했다.
탄소-수소 결합 활성화를 통한 유기물 촉매 개발은 의약품이나 산업 공정에 활용될 수 있는 주요 연구 분야 중 하나다. 특히 생체 모사 연구에서 다양한 금속효소의 활성을 모방해 경제적인 금속 촉매를 만들고자 노력 중이다.
최근 금속 효소 중 철, 망간 등 전이금속과 플루오린과 같은 할로젠 원자가 결합된 금속-할라이드 물질이 다양한 유기물을 산화시키는 중간체로 주목받고 있다.
연구팀은 망간-플루오린 촉매를 새롭게 합성했다. 그동안 보고된 금속-할라이드 종들 중 가장 반응성이 높았다. 반응성이 높아지면 강하게 겹합된 원자들을 분해해 다른 화합물로 전환시킬 수 있다. 다양한 산업 공정에서 무궁무진하게 사용될 수 있는 것이다.
연구팀은 개발된 촉매가 어떻게 산화 반응을 시키는지에 대한 원리를 분석했다. 개발된 촉매로 각종 화합물의 전자적인 환경을 조절해 변화된 반응속도를 확인했다. 특히, 새로 개발된 촉매는 높은 효율로 톨루엔 유도체를 산화시킬 수 있었다. 기존 금속-할라이드 종에선 관찰할 수 없었던 반응이다.
톨루엔 유도체는 유기 물질로 높은 농도로 노출될 경우 건강에 부정적인 영향을 미칠 수 있는 유독성 물질이다. 개발된 촉매를 활용하면 톨루엔 유도체가 산화되며 더 독성이 약한 화합물로 변화하는 것을 확인한 것이다.
제 1저자 정동현, 이유정 연구원은 “이번 연구는 전이금속-플루오린 종의 물리 화학적 특성을 보고한 첫 연구이며, 전자 전달 반응을 기반으로한 탄소-수소 결합 분해 원리를 새롭게 제시했다”고 전했다.
조재흥 교수는 “전이금속-플루오린 종의 높은 산화 능력을 증명한 것에 있어 특히 학술적 의미가 크다”며 “합성 화학 뿐 아니라 환경 및 산업 분야에서도 중요한 금속 촉매 개발에 도움이 될 수 있을 것”이라고 전망했다.
이번 연구는 조재흥 교수 연구팀 정동현, 이유정 연구원이 공동 1저자로 참여했다. 연구 결과는 화학분야 저명 국제학술지인 ‘미국화학회지(JACS, Journal of the American Chemical Society)’에 2월4일 온라인 게재됐다.
연구 수행은 과학기술정보통신부 한국연구재단이 주관하는 단계도약형 탄소 중립 기술 개발사업, 학문 후속 세대 지원사업, DACU 원천기술개발 (R&D), 국가 신약 개발사업 지원으로 이뤄졌다.
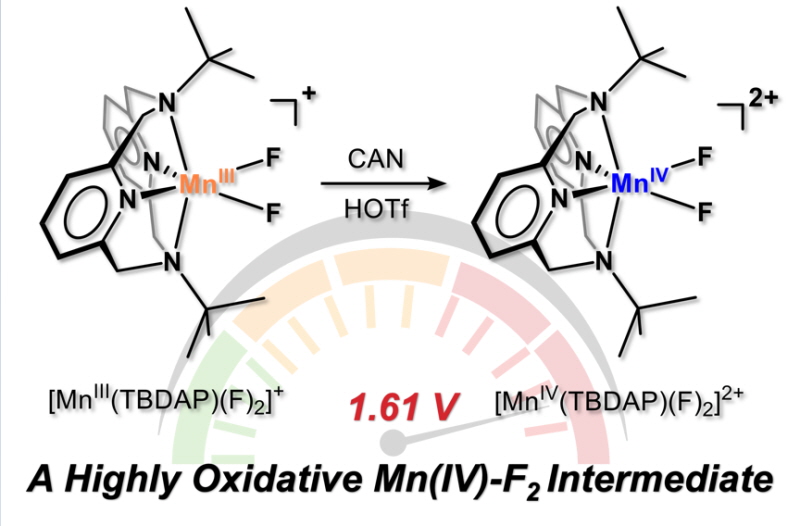
▲1.61 V의 높은 환원 전위를 지닌 고원자가 망간-플루오린 종의 합성 도식

