UNIST는 에너지화학공학과 이현욱 교수팀이 배터리 양극 신소재인 과리튬 소재의 산소 발생 원인을 규명하고 이를 해결할 소재 설계 원리를 제시하며, 향후 장거리 주행 배터리 개발이 가시화 될 것으로 기대된다.
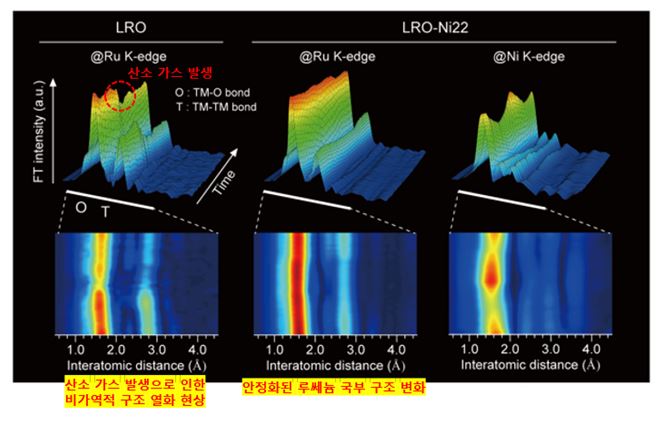
▲니켈 치환으로 안정화된 양극재의 실시간 충·방전 국부 구조 변화 분석
UNIST, 과리튬 양극 소재 산소 가스 발생 억제 전략 제시
과리튬 양극 소재의 산소 가스 발생을 억제해 폭발 걱정 없이 1,000㎞를 주행할 수 있는 배터리를 만들 수 있는 기술이 개발돼 향후 장거리 주행 배터리 개발이 가시화 될 것으로 기대된다.
UNIST는 에너지화학공학과 이현욱 교수팀이 배터리 양극 신소재인 과리튬 소재의 산소 발생 원인을 규명하고 이를 해결할 소재 설계 원리를 제시했다고 18일 밝혔다.
과리튬소재는 이론적으로 4.5V 이상의 고압 충전을 통해 배터리에 기존보다 30%∼70% 더 많은 에너지를 저장할 수 있는 소재다.
전기차 주행거리로 따지면 한 번 충전으로 최대 1,000km를 갈 수 있는 셈이다.
반면에 이 소재는 실제 고압 충전 과정에서 소재 내부에 잡혀 있던 산소(O-2)가 산화돼 기체 형태(O2)로 방출되면서 폭발 위험이 커지는 문제가 있다.
연구팀은 4.25V 부근에서 산소가 산화되면서 부분적인 구조적 변형이 발생해 산소 가스가 방출된다고 분석하고, 이 산소의 산화를 원천적으로 막는 전극 소재 설계 방식을 제안했다.
과리튬소재의 전이금속 일부를 전기음성도가 더 낮은 전이금속 원소로 치환하는 전략이다.
두 금속 원소 간 전기음성도의 차이로 전기음성도가 큰 원소 주변으로 전자가 몰리면 전이 금속의 가용 전자 수가 증가해 산소가 산화되지 않는다.
반면에 전이금속의 가용 전자수가 부족한 상황에서는 산소가 전자를 대신 주고 산화돼 기체 형태로 배출된다.
제1 저자인 김민호 UNIST 박사(現 미국 UCLA 박사후 연구원)은 “기존 연구는 산화된 산소를 안정화시켜 기체 형태로 배출되는 것을 막는 데 주력한 반면 이번 연구는 산소의 산화 자체를 막는 데 집중한 것이 차별점”이라고 설명했다.
또한 이 같은 전자 밀도 변화는 유도효과로 충전 전압을 상승시켜 고에너지밀도를 달성할 수 있다.
에너지 밀도는 가용 전자 수와 충전 전압에 비례하기 때문에 전이 금속을 치환하는 전략으로 배터리 단위 무게당 더 많은 에너지를 저장할 수 있게 된다.
댐에 물이 많고 낙차가 클수록 더 많은 에너지가 저장되는 것과 흡사한 원리다.
연구진은 전이금속 치환 전략의 산소 산화 억제 효과를 실험적으로 확인했다.
가속기 기반 X선 분석 결과, 루테늄의 일부를 니켈로 치환한 경우 산소 기체 발생이 월등히 줄었다. 또 밀도 범함수 계산(DFT)을 통해 전하 재배치가 발생함을 이론적으로 입증했다.
이번 연구는 KAIST 서동화 교수, 중앙대학교, 포항가속기연구소, 미국 UCLA 대학 유장 리(Yuzhang Li) 교수, UC버클리대학, 로렌스버클리연구소가 함께했다.
가속기 기반 X선 분석은 중앙대학교 장해성 교수(공동 제1저자)가 맡았으며, DFT 이론계산은 미국 로렌스버클리연구소의 이은렬 박사(공동 제1저자)가 주도적으로 수행했다.
이현욱 교수는 “다양한 실험과 이론분석으로 기술을 라이브러리화해 양극재 연구자들에게 소재 개발 방향성을 제시했다”며 “에너지 밀도를 높인 폭발 없는 장거리 주행 배터리 소재 개발에 도움이 될 것”이라고 말했다.
이번 연구는 한국연구재단의 원천기술 국제협력 개발사업의 지원으로 수행됐으며, 연구 결과는 미국과학협회(AAAS)에서 발행하는 세계적인 권위지 사이언스(Science)의 자매지인 ‘사이언스 어드밴시스(Science Advances)’에 2월19일자로 온라인 게재됐다.